Another location that we obtained dirt from is the Wet Willy's cash wash which is located at 701 New Loudon Rd, Latham, NY 12110. Our intention was to find something interesting compared to the set of elements we generally analyzed. The dirt sample was wet and so to remove all the moisture trapped in the dirt, it needed to be dried up in the oven.
As usual, the dirt sample was analyzed in both the SEM and the HD Prime.
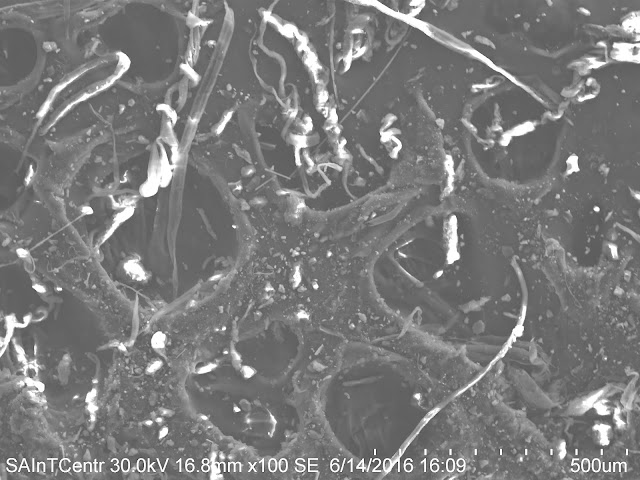
The raw image of the section of the dirt sample that was analyzed in the SEM is shown bellow;
The lighter shade particles that can be seen in the sample above represents the large particles of dirt.
The sample was analyzed for 9 minutes in the EDS to get better results of the element composition. The following results was obtained for the elemental mapping of the sample. The following picture also contains a outline of the raw image shown above. As usual, each element is represented in different colors.
The result that was obtained was more clear than the raw image itself and the small dirt particles can be seen as well. Note that Carbon and Oxygen are neglected in analyzing the sample for elemental compositon.
The new elements that were traced were Cadmium and Manganese.
The map of elemental composition itself is shown;
The spreadsheet data that was obtained after quantifying for weight composition of the dirt sample is shown below;
| Element |
AN |
series |
[wt.%] |
[norm. wt.%] |
[norm. at.%] |
Error in wt.% (1 Sigma) |
| Sodium |
11 |
K-series |
0.517452789 |
1.307357089 |
1.79880694 |
0.062543746 |
| Magnesium |
12 |
K-series |
0.685729394 |
1.732512035 |
2.254787351 |
0.065966061 |
| Aluminium |
13 |
K-series |
5.332921697 |
13.47375672 |
15.79599263 |
0.292948185 |
| Silicon |
14 |
K-series |
22.5837991 |
57.05851916 |
64.26333962 |
1.02922487 |
| Phosphorus |
15 |
K-series |
0.174544453 |
0.440990816 |
0.450360788 |
0.033684536 |
| Sulfur |
16 |
K-series |
0.054706279 |
0.138216748 |
0.136345508 |
0.02809259 |
| Calcium |
20 |
K-series |
2.620305254 |
6.620265123 |
5.225096264 |
0.103002426 |
| Titanium |
22 |
K-series |
0.515898718 |
1.303430691 |
0.861110244 |
0.040437744 |
| Manganese |
25 |
K-series |
0.048569741 |
0.122712636 |
0.070654755 |
0.027262929 |
| Iron |
26 |
K-series |
5.741094516 |
14.5050153 |
8.215675883 |
0.171269138 |
| Cadmium |
48 |
L-series |
1.305043287 |
3.29722369 |
0.927830017 |
0.06695208 |
|
|
Sum: |
39.58006523 |
100 |
100 |
|
The most amount of weight composition that was found in the dirt sample was Silicon with a weight percentage of 57.1%. This is expected to be there because of the sand. A significant amount of Aluminium, Calcium and Iron can be seen with a weight percentage of 13.5%, 6.62% and 14.5% respectively.
The spectrum of the elements that were found in the same sample is shown below;
The amount of elements in the dirt sample can be seen in the spectrum using the peaks.
The results for the data that was obtained from analyzing the sample in the HD Prime is shown below.
Recall from the previous blog post...
...The cam view (wide view, Side view) of the sample is included in the data. The row of elements after the view shows the common elements that are expected to be seen in the sample. However to get a accurate results, we should look into the full results table shown in the second image. We will be looking into the counts column in this part to identify the elements with the most amount of counts that were detected...
Using the HD Prime, a excessive amount of Chromium was seen to be found using the list of elements shown after the side/wide view. This is seen using the red outline in the general list of elements that is found. To confirm this first look, we can look into the full results that can be seen in the second image.
611.9 counts were measured for Chromium which is not a lot but still a consistent amount for a heavy metal.
Chromium is a lustrous, brittle, hard metal. Its color is silver-gray and it can be highly polished. It is mainly used in stainless steel, chrome plating and metal ceramics. It is also used to impart corrosion resistance and a shiny finish; as dyes and paints, its salts color glass an emerald green and it is used to produce synthetic rubies; as a catalyst in dyeing and in the tanning of leather.
Hence finding it in a Car wash can be completely understood but still a frequent level of exposure is advised to be avoided.
Other elements such as strontium, Iron, Copper, Titanium and Zinc was analyzed to be in the sample with counts exceeding 1500 counts. However these elements are not known to be hazardous.
References;
http://www.lenntech.com/periodic/elements/cr.htm